
Distinguishing features include the large globular luminal domain of Nicalin (in contrast with the flexible luminal domains of NOMO and CCDC47), the large globular cytosolic domain of CCDC47 (with a conserved C-terminal coiled-coil), and a conserved cytosolic coiled-coil between the first two TMDs of TMCO1. ( D) Topology and domain structure for the top hits, based on Uniprot annotation, homology modeling, de novo structure prediction (in RaptorX-Contact), and experimental mapping the Sec61 complex is not shown. The catalytic STT3A subunit of the OST complex is not detected. ( C) Top hits were confirmed by western blotting. ( B) Proteins enriched in the ribosomal fraction after immunoprecipitation from 3xFlag-TMCO1 or wild-type membranes. ( A) Emetine- and micrococcal nuclease-treated membranes from wild-type (WT) or 3xFlag-TMCO1 (Flag) HEK293 cells were digitonin-solubilized, immunoprecipitated via the 3xFlag tag on TMCO1, and the eluate sedimented through a sucrose cushion to isolate the ribosome-associated fraction for analysis.
Additionally, it suggests that human diseases caused by mutations in TMCO1 result from a defect in the production of multi-pass membrane proteins. This work provides a framework for understanding how these proteins are correctly made in the membrane. reveal a previously unknown cellular machinery which may be involved in the production of hundreds of human multi-pass proteins. The experiments revealed that the translocon is required for the production of a multi-pass protein called EAAT1, and it provides multiple ways for proteins to be inserted into and folded within the membrane. showed that the translocon interacts with ribosomes that are synthesizing multi-pass proteins. Using a combination of biochemical, genetic and structural techniques, McGilvray, Anghel et al. discovered that TMCO1, together with other proteins, is part of a new ‘translocon’ – a group of proteins that transports proteins into the endoplasmic reticulum membrane. – including several of the researchers involved in the 2017 study – wanted to determine what TMCO1 does in the cell and begin to understand its role in human disease. TMCO1 has been linked to glaucoma, and mutations in it cause cerebrofaciothoracic dysplasia, a human disease characterized by severe intellectual disability, distinctive facial features, and bone abnormalities. A study from 2017 showed that a protein called TMCO1 is related to a group of proteins involved in making membrane proteins.

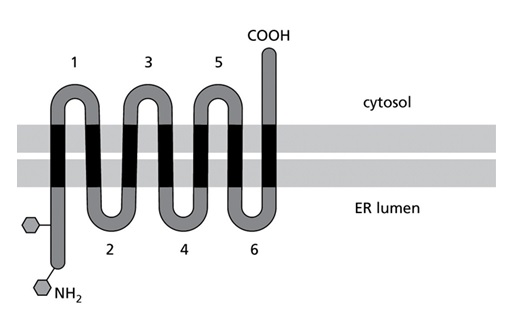
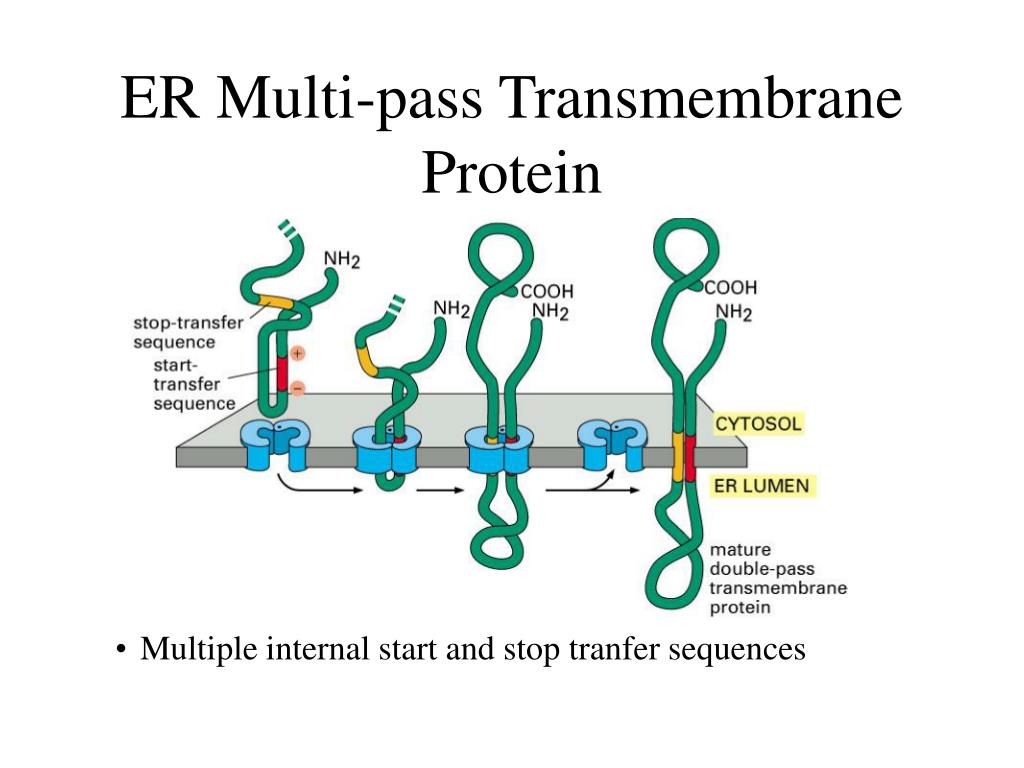
Despite decades of work, however, much less is known about how multi-pass proteins that span the membrane multiple times are made. This process is best understood for proteins that span the membrane once. As the new protein is made by the ribosome, it enters the endoplasmic reticulum membrane where it folds into the correct shape.
Transmembrane proteins are synthesized by ribosomes – protein-making machines – that are on the surface of a cell compartment called the endoplasmic reticulum. Nearly 25% of human genes encode transmembrane proteins that span the entire membrane from one side to the other, helping the membrane perform its roles. These results identify a new human translocon and provide a molecular framework for understanding its role in multi-pass membrane protein biogenesis.īiochemistry biogenesis cell biology chemical biology co-translational endoplasmic reticulum folding human human disease insertion multi-pass membrane protein ribosome translocon.Ĭell membranes are structures that separate the interior of the cell from its environment and determine the cell’s shape and the structure of its internal compartments. Consistent with a role in multi-pass membrane protein biogenesis, cells lacking different accessory components show reduced levels of one such client, the glutamate transporter EAAT1. High-throughput mRNA sequencing shows selective translocon engagement with hundreds of different multi-pass membrane proteins.
Similar to protein-conducting channels that facilitate movement of transmembrane segments, cytosolic and luminal funnels in TMCO1 and TMEM147, respectively, suggest routes into the central membrane cavity. Cryo-electron microscopy reveals a large assembly at the ribosome exit tunnel organized around a central membrane cavity. Here we describe a ~ 360 kDa ribosome-associated complex comprising the core Sec61 channel and five accessory factors: TMCO1, CCDC47 and the Nicalin-TMEM147-NOMO complex. Membrane proteins with multiple transmembrane domains play critical roles in cell physiology, but little is known about the machinery coordinating their biogenesis at the endoplasmic reticulum.


 0 kommentar(er)
0 kommentar(er)
